Non-invasive cardiac assessment company Sensydia announced today that it has completed its 50-subject development study at the University of Minnesota (UMN). This study was conducted at UMN to collect data for its innovative AI-powered, non-invasive Cardiac Performance System (CPS™) that uses heart sound analysis to enable earlier detection and more effective therapy guidance for patients suffering from heart failure and pulmonary hypertension.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240116959632/en/
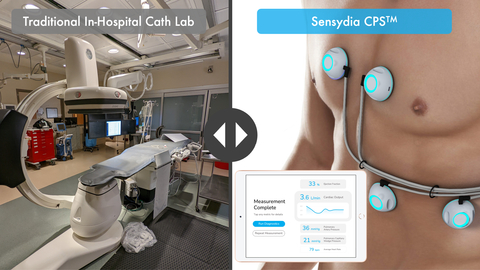
Sensydia’s Cardiac Performance System (CPS™) is designed for fast, safe, and non-invasive cardiac performance assessment that can be performed almost anywhere, avoiding the need to visit a catheterization lab. Source: Sensydia.
Sensydia is developing the CPS platform, which uses ultra-sensitive biosensors to provide clinicians with rapid, non-invasive measurement of ejection fraction, cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure in a handheld device. Knowledge of these parameters is critical when caring for individuals with heart failure and pulmonary hypertension. To obtain these measurements today, patients must undergo echocardiography and invasive right heart catheterization, which are resource intensive, restricted to medical facilities, and only provide snapshot data. In contrast, CPS measurements are fast, safe, may be repeated as frequently as indicated, and can be performed essentially anywhere with minimal training.
“This is Sensydia’s fifth successful study, and we will continue to collect data across leading cardiac care institutions to improve the performance and utility of the artificial intelligence algorithms that power our breakthrough CPS platform,” said Anthony Arnold, President and CEO of Sensydia. “We’re working to overcome today’s barriers in acquiring vital hemodynamic measures for monitoring and managing cardiac patients, and we appreciate the participation of cardiologists and staff at University of Minnesota in working toward this goal.”
“The CPS platform shows promise as a non-invasive alternative to routine echocardiography and right-heart catheterization. It has the potential to positively impact the way patients with heart failure are monitored, managed, and ultimately to improve clinical outcomes,” said Tamas Alexy, MD, Associate Professor of Medicine and Principal Investigator, UMN Medical School. “These measurements are essential in the diagnosis and ongoing management of patients with heart failure as well as pulmonary hypertension. We are pleased to help evaluate Sensydia’s platform utilizing acoustic sensing technology and advanced AI algorithms, reducing the need for repeat echocardiograms and invasive hemodynamic assessments.”
Sensydia previously conducted a study at the Ronald Reagan UCLA Medical Center which contributed to the FDA clearance for its ejection fraction algorithm, a study at the OHSU Knight Cardiovascular Institute for its cardiac output algorithm, and two studies at the University of Pittsburgh Medical Center for its pulmonary pressure algorithm and general clinical utility.
In January 2022, Sensydia announced that CPS was granted Breakthrough Device Designation by the United States Food and Drug Administration (FDA). Sensydia plans to use data from this 50-subject study to develop the CPS pulmonary pressure algorithms.
The achievement of this 50-subject milestone comes at a time when Sensydia is actively executing Phase I of its new NIH award. With its CPS device, Sensydia aims to make non-invasive, fast, and safe cardiac assessment broadly available to improve the life of those living with heart failure and pulmonary hypertension.
About Sensydia
Sensydia intends to revolutionize heart failure therapy by expanding access to cardiac performance assessment outside the hospital with its non-invasive Cardiac Performance System (CPS™). Today the U.S. spends more than $30 billion annually on the diagnosis and management of heart failure, which requires costly and risky in-hospital catheterization procedures to obtain an accurate assessment of cardiac performance. Sensydia’s CPS platform delivers accurate, non-invasive assessment of cardiac performance (cardiac output, ejection fraction, and pulmonary pressures) almost anywhere in under 5 minutes. CPS utilizes proprietary waveform machine learning methods that have been trained against gold-standard measurements from in-hospital catheterization lab data.
In 2018, Sensydia received FDA 510(k) clearance for non-invasive measurement of ejection fraction using first-generation hardware. Sensydia is on a mission to improve the quality of human life by democratizing health assessment and is committed to serving the needs of patients and healthcare providers by delivering non-invasive and easy-to-use products. Learn more at sensydia.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240116959632/en/